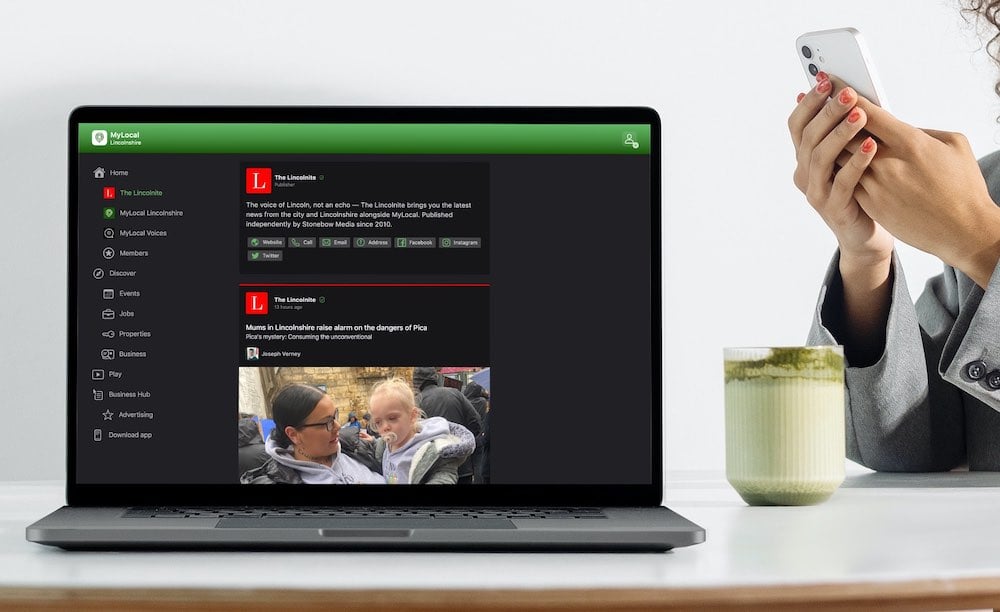
Exciting news for MyLocal app users and The Lincolnite readers! Our latest update is now available to download from your app store and comes packed with the best local news experience you can enjoy.
Discover MyLocal: Download the app today for iPhone or Android
Download now to enjoy:
- Revamped News Feed: Enjoy faster loading times and smoother scrolling that make catching up on local news a breeze.
- New Play Video Feed: Dive into a dynamic video feed that brings local stories to life in a whole new way.
- Enhanced In-App Browser: Access external links quickly and efficiently, right from the app, without any distractions.
Update your MyLocal app from your app store (version 2.73) now and start enjoying these great new features today!
P.S.: Start your MyLocal membership today to support MyLocal and The Lincolnite deliver you a better local news experience and honest journalism from across Lincolnshire.
P.P.S.: We have some huge things in the pipeline, stay tuned!

 Whatsapp
Whatsapp